The New RSV Shot for Infants
By semantic sleight of hand, the CDC’s new RSV shot for infants is called ‘not a vaccine, because it’s a monoclonal antibody.’ Here’s why that argument is false. And FDA evidence of the hazards.
A little background on why I am writing this
First, I would like to clarify that I am a long-term fan of RFK Jr, and I hope he will fulfill his mission – the toughest job I can imagine - to stop total control of all human health by the pharmaceutical industry, and that he will help guide evaluation of vaccines on their merits or lack thereof. I have written medical exemptions from vaccines for patients since parents first requested them from me, nearly 20 years ago, and my clinic has never refused a request for medical exemption, from anywhere in the world. And in full disclosure, I have been anti-vax so long that I even have some, but not all, Amish ancestry, and I remember way back decades ago when the best-known critic of vaccines in the U.S. was Dr. Tedd Koren, whom no one else seems to mention these days. So I can never be grateful enough for Secretary Kennedy’s leadership of HHS, because he is the first one heading HHS to resist pharma’s near omnipotence.
However, the CDC’s Advisory Committee on Immunization Practices (ACIP) needs some work, shall we say. Here’s why.
In late June 2025, ACIP voted 5 to 2 to approve a monoclonal antibody injection (MAB) for all healthy infants, intending to prevent respiratory syncytial virus (RSV).
This vote to approve the shot happened even after one of its members, MIT professor Retsef Levi PhD revealed to the Committee that previous use of monoclonal antibodies in infants to prevent RSV killed more infants than it saved, as I will show below. He showed the data of those infants’ deaths from the clinical trials, which I show in his words, and from clinical trial sources below. Yet the ACIP Committee then voted to approve the injection, even after having heard that very alarming history. So we have a problem that needs our urgent attention.
A deep dive into the MABs for RSV is warranted in order to help the ACIP Committee to understand and to reverse their mistake, and for the public to keep up the pressure for the new CDC / ACIP to do right by their number one constituency – the American public, kids and adults - and NOT to bend the knee to the pharmaceutical industry, as their predecessors did..
But first, let’s look at a brief history of RSV and prior efforts to prevent it.
RSV virus itself has a concerning history
Chanock and Finberg reported in 1957 the escape of a respiratory virus from chimpanzees to humans that then became known as respiratory syncytial virus (RSV). It was indistinguishable from chimpanzee coryza virus. [1] It was then observed to contaminate polio vaccines. [2] After that, widespread exposure was observed in the world population. By age two, every child is assumed to have been exposed to RSV. It is estimated that 1 out of every 100 children living in the EU are hospitalized due to RSV each year. [3]
RSV vaccines also have a concerning history
In the 1960’s, the RSV-vaccinated infants later became infected with RSV as often as the RSV-unvaccinated, but the vaccinated who got infected had an 80% chance of being hospitalized with it, with severe infection, compared to 5% of the unvaccinated, and those hospitalized developed pneumonia and bronchiolitis. Of those groups, 69% of the vaccinated developed pneumonia, but only 9% of the unvaccinated developed pneumonia. [4] Unfortunately, some of that data are behind a paywall. [5] [6] Simöes wrote in 1999, now paywalled [7] but quoted in: [8] “RSV-naïve infants who received formalin-inactivated RSV vaccine, and who were naturally infected with RSV later, developed more severe disease in the lower respiratory tract than a control group immunized with a trivalent parainfluenza vaccine.” Pfizer was the manufacturer. Pneumonia and bronchiolitis can be life-threatening in infancy.
That vaccine claimed the lives of infants due to an exaggerated Th2 immune response with non-neutralizing antibodies. That process is known as original antigenic sin or antibody-dependent enhancement. The result of that process is accumulation in the respiratory tract of enough useless, misdirected antibodies and eosinophils to produce illness, without the right kind of antibodies to oppose the infectious pathogen. [9]
Why there cannot be a safe and effective RSV vaccine
RSV vaccines cannot be made well for a few reasons. One is that RSV is a RNA virus, which, like coronaviruses such as SARS-CoV-2, is fleeting and will not stand still long enough genetically speaking, to wait for development, manufacture and distribution of a mass-produced vaccine. It is like hitting a fast-moving fly with a slow fly swatter. New, mutated RSV viruses will very soon be resistant to any vaccine or injected antibodies that were manufactured some months earlier and later injected at a “well child visit.” This is one reason why RNA viruses have always been notoriously resistant to vaccine prevention, and the fixed protein monoclonal antibodies are no better, for their unchanging form during the development-mass production-distribution process.
Another defect of RSV vaccines is that heat and formalin (a known carcinogen) have been used to inactivate a virus for vaccines. As with other vaccines, this process is hard to control, [10] has resulted in an off-target protein, the post-fusion protein, whereas the natural infection confers each of the proteins, pre-fusion and post-fusion, with the pre-fusion considered to be the better situated for having opposing antibodies.
And then there is the compartment problem, which I discuss later on.
What is a vaccine?
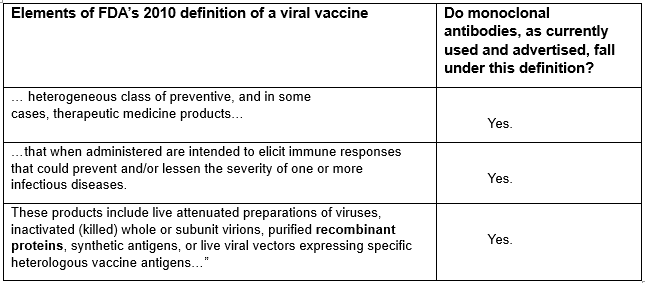
The FDA defined a “viral vaccine” in 2010 [11] as follows:
“For the purpose of this document, viral vaccines are a heterogeneous class of preventive, and in some cases, therapeutic medicinal products that when administered are intended to elicit immune responses that could prevent and/or lessen the severity of one or more infectious diseases. These products include live attenuated preparations of viruses, inactivated (killed) whole or subunit virions, purified recombinant proteins, synthetic antigens, or live viral vectors expressing specific heterologous vaccine antigens…”In light of the above definition, and its component elements, let’s now examine the claim of a member of the CDC’s ACIP vaccine approval committee, that “monoclonal antibodies are not vaccines.” Here is his explanation of that remark.
This is an interesting claim, also because he is a member of the CDC’s ACIP Committee, and it is a widely held usage of “immunizations” and “vaccines” to be synonyms. Therefore, there must have been something vaccine-like about the monoclonal antibody substance being voted on by that Committee, to have considered it in their “immunization” wheelhouse, and to have voted on it.
But there is another claim here that has long been disproven. Dr. Malone claims that injected proteins do not “teach” the immune system to remember the threat.
Actually, injected proteins, including antibodies, like other foreign proteins, are antigenic in humans. Foreign proteins have created enormous long-term health consequences for people, simply as being adjuvants of vaccines, and have trained immune systems in many undesirable directions, such as peanut allergies from peanut oil excipients, and egg allergies, from culturing vaccines on chick embryos. So then we are left with some of the most vexing problems confronted by over-vaccinated populations – their daily dealing with allergies and other autoimmune challenges. This is now conclusively understood to be a consequence of vaccine ingredients, as shown in the enormous Immune Epitope Database.[12] RFK Jr. has commented that no one he knew growing up had a peanut allergy. “Why do three of my kids have peanut allergies?” It’s the excipients used in the vaccines that they had years ago. Those are proteins, and antibodies are proteins. Injected proteins enter the blood by an unnatural route, and arriving there, they provoke our own persistent antibody and other immune assaults against them.
So Robert Malone’s claim that injected antibodies do not “teach” the immune system does not hold water. For many people their prior vaccines, including foreign proteins, have “taught” the immune system all too well the wrong lessons.
Do monoclonal antibodies function as vaccines?
Now let’s look at the above definition of a viral vaccine, and see if the monoclonal antibody, used with the intention to prevent an infectious disease, meets all the criteria of that FDA definition of a “viral vaccine.”
In sum, considering all of the criteria above, do monoclonal antibodies satisfy each of the above elements of the definition of a vaccine? I think that they do.
The CDC does not seem to define vaccine directly, but it does define immunization. “Immunization is the process of being made resistant to an infectious disease, usually by means of a vaccine.” [13]
The process of “being made resistant to infectious disease” seems to encompass the advertisements and rationales for monoclonal antibodies, including the newly recommended shot for all infants, the clesrovimab injection, a monoclonal antibody that is advertised to help prevent respiratory syncytial virus (RSV).
Monoclonal antibodies generally
Monoclonal antibodies are taken from B-cells, from one of several sources: Either human fetal cells, such as the HEK line, that is the human embryonic kidney cell line, number 293. That is, it is said that the kidneys of the first 292 fetuses did not “take,” for this purpose anyway, so it was fetus number 293 from which so many of these cell lines are cultured. [CORRECTION (7/5/25): It is said that 293 derives from the number of experiments, and it is not clear from the researcher's 1970s notebook if the HEK 293 cell line was derived from many or fewer fetuses involved in that series of experiments. I have not seen that notebook.] This is the same cell line used in developing the Moderna and Astra Zeneca vaccines.
Those babies are aborted while still living, with the heart still beating and pumping blood to the kidneys, so that the harvest of the fetal kidneys will “work” for their harvesting purposes. Attorney Aaron Siri who litigates these issues describes some of the gruesome process here: https://x.com/newstart_2024/status/1941215682026405910
Two other sources are mice and rats.
There are also human and mouse genome material combined inside mouse cells. They call the resulting hybridized B-cells’ antibodies a chimera source antibody.
And then there is an even more threatening source: cancerous cells. Hybridoma technology fuses B cells, which produce antibodies, to immortal myeloma cells (yes, as in multiple myeloma cells). All myeloma is cancerous.
In the case of clesrovimab, the newly approved shot, it is “derived from human parental antibody RB1,” [14] where RB stands for retinoblastoma, which is a cancer of the eye in children, especially children younger than age 5. [15] The RB1 is thought of as a tumor suppressor gene. However, huge production of B cells is needed for large scale manufacture of widely deployed injections. Cancerous origins of B-cells are much more proliferative than from normal cells and normal tissue origins, and may suit large industrial demand adequately compared to normal tissue origin. Mutations in RB1 lead to retinoblastoma.
Perhaps the biotech specialists handling these materials make exactly the right choices to not allow a promotion of cancer through the cell lines they work with to harvest antibodies. Perhaps they are supremely knowledgeable and careful to not let that happen. And then again, maybe these experiments are just too risky and way too early to subject human children to at this time (or ever). And this RB1 is what is being used to produce clesrovimab, which is what the CDC’s ACIP just approved for injection into all healthy infants!
Failures in monoclonal antibody development for RSV
Just as with the earlier vaccines, monoclonal antibody (MAB) development is acknowledged to miss its mark, of targeting antibodies to the desired protein in earlier attempts to create a monoclonal antibody for RSV.
The respiratory syncytial virus (RSV) has a key protein called the fusion glycoprotein, or simply F protein. It is thought to control the fusion of the RSV virus with a human cell membrane, [16] allowing entry into the human cell, one of the most decisive steps in the process of infection.
Repeated experimental failures to meet that objective of targeting that protein with antibodies led authors to conclude, “This indicates that the loss of recognition of F1-RSV by pre-F specific antibodies is not due to chemical modifications by formalin, but rather due to modifications of pre-F conformation.” [17] In other words, the fluid and rapid mutations of mRNA viruses and their associated proteins obviate the potential effect of any slow fly-swatter approach. Newly mutated RSV viruses will inevitably resist these injected antibodies, because they are different enough to escape that targeting.
Clesrovimab hazards
Regarding clesrovimab, brand name Enflonsia, which the CDC approved in June 2025 for infants, Pfizer and Merck authors claim that there are no increases in morbidity or hospitalization of MAB-injected infants over controls. [18] [19] However, it would have been helpful to know what they included in the injection that they asserted to be a “placebo.”
That claim for safety of clesrovimab turned out to be false, as reported on ClinicalTrials.gov, and shown here: [20]
The above chart shows a total of 4 infant deaths, with one total in the placebo group. Dividing by participants in each group = 0.16% risk of dying in the treatment group and 0.08% risk of dying in the placebo group. That leaves the clesrovimab infants with roughly twice the risk of dying. I say rough due to (thankfully) small numbers.
The following part of the chart on ClinicalTrials.gov shows quite a bit more gastroenteritis in the clesrovimab group.
Proportionally to participants, that’s about a 50% increase in the rate of gastroenteritis for the clesrovimab-jabbed infants.
The other monoclonal antibody for RSV also had bad results: Nirsevimab trials
Results of the similar MAB for RSV, known as nirsevimab, brand name Beyfortus, had lethal results for newborns in France in 2023 to 2024. The results showed “a significant signal of an increase in newborn deaths between 2 and 6 days of age during the 2023-2024 immunization [nirsevimab] campaign.” [21]
Of the 153 serious cases observed in France, 75% of the reported cases involved bronchiolitis after injection. [22]
Here is a table of the effects of nirsevimab in treated group vs placebo group, shown in the “Argument . . .” column, as arguing for a causative role in antibody-dependent enhancement (ADE) disease results. [23] URTI is upper respiratory tract infection. LRTI is lower respiratory tract infection, which the reader likely knows to be clinically much more risky than a URTI.
The FDA also found a 45% higher death rate in nirsevimab treated infants compared to control infants. [24] Here are the FDA’s reported data on that:
The last line shows the MAB treated infants had 0.32 / 0.22 = 1.45 times the deaths of the control infants.
In another study of nirsevimab, the “Melody Trial,” the authors reported four deaths in the treatment cohort. [25] However, Prof. Levi also uncovered a fifth death, buried in a footnote in an appendix. The note explained that that 5th death was excluded for being on Day 440, which was later than the 361 days of the observation period. [26] However, elsewhere the safety follow-up period was specified to be 510 days. (Thanks to Yaffa Shir-Raz for showing these details in the published literature.) [27] Dr. Levi expressed alarm over these findings on March 4, 2025, and it is worth re-printing his warnings here: https://x.com/RetsefL/status/1896971108517339490
The EudraVigilance database shows reports of adverse events in clinical settings for nirsevimab. It is comparable to the VAERS system in the U.S. “By April 15, 2024, there were 140 reports, mainly from healthcare professionals (138/140), 89 of which were for ‘bronchiolitis,’ 129 for ‘RSV’ and 56 for ‘drug ineffective.’ Only 26 reports did not concern respiratory events.” [28] [29]
The half-life of the clesrovimab antibodies is 45 days, [30] which is a lot longer than for most of the other monoclonal antibodies, but still should be disclosed to parents, in weighing the huge risk of these shots given the short time of antibody presence detected.
The compartment problem
Monoclonal antibodies cannot prevent respiratory infection. That’s because they are a blood based IgG isotype. It is essentially a compartment problem. IgG antibodies don’t leave the blood to patrol the airway surfaces, as natural secretory IgA and many other immune system cells do.
Blood does not normally enter the lungs or the airways. The only exceptions to that are blunt force trauma piercing the lung, extreme exertion such as sustained fast running or lung cancer. Other than those unusual experiences, blood is separated by respiratory tissues, from nasal mucosa on down to deepest alveoli of the respiratory tract, and that is where respiratory syncytial virus would enter and be fought.
The compartment problem has been understood since at least 1940, described by T Francis. [31] I explain it with respect to vaccines’ lack of efficacy. [32] But if the compartment issue were at all widely understood, even if it were understood by members of CDC’s ACIP Committee, then the RSV shot would not have been approved. And four years ago the COVID vaccines would have been understood to be useless before they were inflicted on five billion people, and decades ago the flu shot would have been understood to be useless, prior to decades of injectees discovering that fact the hard way.
Vitamin D is the best protection against RSV
Despite all the frenzied biotech activity, the best preventative of RSV remains vitamin D. Infants born with low vitamin D levels, below 50 nmol/L were 6 times as likely to develop RSV infection in their first year of life as those babies with > 75 nmol / L in the blood. [33]
Even the claimed benefits of MAB to prevent RSV come nowhere near that six to one ratio of RSV infection risk.
It is no small thing to make sure that the mom of the breastfeedlng infant gets adequate vitamin D. RSV has been the number one cause of hospitalization in children under the age of 5. [34]
Ivermectin has four decades of safety studies supporting its use, and it is considered to be one of the safest drugs in use. [35] Effectiveness of ivermectin against SARS-CoV-2, another RNA virus, is now well-established throughout the world, [36] despite demurring comments from the pharmaceutical industry.
As it turns out, ivermectin has shown effect in vitro against bovine respiratory syncytial virus. [37] It is a known inhibitor of the importin α/β protein that carries a virus into a cell nucleus for replication. I look forward to learning future results about use of ivermectin therapeutically against human RSV also.
As for the monoclonal antibodies, on the other hand, to be of benefit, they should reduce hospitalization, and reduce admission to intensive care, and reduce deaths. So far, the monoclonal antibodies for RSV have increased those disasters. As Dr. Levi asked at the end of his above-shown tweet: “Does this sound like the reassuring level of safety & efficacy for a drug given to healthy young infants?”
Therefore, the ACIP Committee should reverse their approval of clesrovimab urgently, before more infants are shot with it.
[1] R Chanock, B Roizman, et al. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). Jul 2 1957. Am J Epidemiology 66 (3). 281-290. https://doi.org/10.1093/oxfordjournals.aje.a119901
[2] V Scheibner. Polio eradication: a complex end game. Aug 26 2012. BMJ. 344. https://www.bmj.com/content/344/bmj.e2398/rr/599724
[3] M Del Riccio, P Spreeuwenberg, et al. Burden of respiratory syncytial virus in the European Union: estimation of RSV-associated hospitalizations in children under 5 years. May 29 2023. 228 (11). 1528-1538. https://pmc.ncbi.nlm.nih.gov/articles/PMC10681872/
[4] A Kapikian, T Mitchell, et al. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Aug 1968. Am J Epidemiology. 89 (4). 405-420. https://watermark.silverchair.com/89-4-405.pdf
[5] H Kim, J Canchola, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Apr 1969. Am J Epidemiology. 89 (4). 422-434. https://academic.oup.com/aje/article-abstract/89/4/422/198849
[6] V Fulginiti, J Eller, et al. Precipitated respiratory syncytial virus vaccine. Apr 1969. Am J Epidemiology. 89 (4). 435-448. https://academic.oup.com/aje/article-abstract/89/4/435/198864
[7] E Simoes. Respiratory syncytial virus infection. Sep 4 1999. 354 (9181). 847-852. https://www.sciencedirect.com/science/article/abs/pii/S0140673699800403
[8] V Scheibner. Polio eradication: a complex end game. Aug 26 2012. BMJ. 344. https://www.bmj.com/content/344/bmj.e2398/rr/599724
[9] A Rigter, I Widjaja, et al. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. Aug 12 2013. PLoS One. 8 (8). https://pmc.ncbi.nlm.nih.gov/articles/PMC3741363/
[10] B Murphy, E Walsh. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. Aug 1988. J Clin Microbiol. 26 (8). 1595-1597. https://pmc.ncbi.nlm.nih.gov/articles/PMC266671/
[11] FDA. Guidance for industry. Feb 2010. FDA.gov https://www.fda.gov/media/78428/download
[12] V Arumugham. Analyzing 23,000+ epitopes covering 82 autoimmune diseases in the Immune Epitope Database; 57% have only one and 78% have up to two amino acid residue differences compared to animal, fungal or plant peptides present in vaccines; an unmistakable role of vaccines in their etiologies.. Jan 2020. PDMJ. https://pdmj.org/papers/epitope_analysis
[13] CDC. Explaining how vaccines work. Aug 10 2024. https://www.cdc.gov/vaccines/basics/explaining-how-vaccines-work.html
[14] S Madhi, E Simoes, et al. A phase 1b/2a trial of a half-life extended respiratory syncytial virus neutralizing antibody, clesrovimab, in healthy preterm and full-term infants. Nov 27 2024. J Infect Dis. 231 (3). e478-e487. https://pmc.ncbi.nlm.nih.gov/articles/PMC11911779/
[15] National Eye Institute. Retinoblastoma. NIH. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/retinoblastoma
[16] J McLellan, W Ray, et al. Structure and function of RSV surface glycoproteins. Oct 28 2014. Curr Top Microbiol Immunol. 372. 82-104. https://pmc.ncbi.nlm.nih.gov/articles/PMC4211642/
[17] A Killikelly, M Kanekiyo, et al. Pre-fusion F is absent on the surface of formalininactivated respiratory syncytial virus. Sep 29 2016. Science Rep. 6. 34108. https://pmc.ncbi.nlm.nih.gov/articles/PMC5040956/#b5
[18] H Zar, E Simoes, et al. 166. A Phase 2b/3 study to evaluate the efficacy and safety of an investigational respiratory syncytial virus (RSV) antibody, clesrovimab, in healthy preterm and full-term infants. Jan 29 2025. Open Forum Infect Dis. https://pmc.ncbi.nlm.nih.gov/articles/PMC11777356/
[19] S Madhi, E Simoes, et al. A phase 1b/2a trial of a half-life extended respiratory syncytial virus neutralizing antibody, clesrovimab, in healthy preterm and full-term infants. Nov 27 2024. J Infect Dis. 231 (3). e478-e487. https://pmc.ncbi.nlm.nih.gov/articles/PMC11911779/
[20] Merck, Sharp & Dohme. Efficacy and safety of clesrovimab (MK-1654) in infants (MK-1654-004) (CLEVER). May 5 2025. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04767373
[21] H Banoun. Independent analysis of the results of the first infant immunization campaign with Beyfortus (nirsevimab, monoclonal antibody against RSV bronchiolitis virus): Mixed results, identification of biases, and possible role and mechanisms of ADE (antibody dependent enhancement). Jun 11 2024. Qeios. https://www.qeios.com/read/PQWEBF#cugFyFg4IWDg
[22] H Banoun. Analysis of the ANSM (French Pharmacovigilance Agency) report on Beyfortus published on September 30, 2024. Sep 30 2024. Research Gate. https://www.researchgate.net/publication/384752319_Analysis_of_the_ANSM_French_Pharmacovigilance_Agency_report_on_Beyfortus_published_on_September_30_2024
[23] H Banoun. Analysis of Beyfortus (nirsevimab) immunization campaign: Effectiveness, biases, and ADE risks in RSV prevention. Sep 2024. Curr Issues Mol Biol. 46 (9). 10369-10395. https://pmc.ncbi.nlm.nih.gov/articles/PMC11431526/
[24] FDA Biologics License Application (BLA) 761328 Nirsevimab Antimicrobial Drugs Advisory Committee Meeting. Jun 8 2023. Division of Antivirals, Office of infectious Diseases Center for Drug Evaluation and Research, accessed Jun 30 2025. FDA. P. 70 https://www.fda.gov/media/169322/download
[25] L Hammitt, R Dagan, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. Mar 2 2022. N Engl J Med. 386 (9). 837-846. https://www.nejm.org/doi/full/10.1056/NEJMoa2110275
[26] W Muller, S Madhi, et al. Supplement to Nirsevimab for prevention of RSV in term and later-preterm infants. 2023. N Engl J Med. 388. 1533-1534. https://www.nejm.org/doi/suppl/10.1056/NEJMc2214773/suppl_file/nejmc2214773_appendix.pdf
[27] Y Shir-Raz post on X. https://x.com/YaffaRaz/status/1939666365431828924
[28] H Banoun. Ibid.
[29] Eudravigilance European database of suspected adverse drug reaction reports. https://www.adrreports.eu/fr/eudravigilance.html. Accessed Apr 15 2024.
[30] S Madhi, E Simoes, et al. A phase 1b/2a trial of a half-life extended respiratory syncytial virus neutralizing antibody, clesrovimab, in healthy preterm and full-term infants. Nov 27 2024. J Infect Dis. 231 (3). e478-e487. https://pmc.ncbi.nlm.nih.gov/articles/PMC11911779/
[31] T Francis. The inactivation of epidemic influenza virus by nasal secretions of human individuals. 1940. Science. 91: 198-199.
[32] C Huber. Respiratory vaccines cannot work. Mar 13 2023. The Defeat of COVID. https://colleenhuber.substack.com/p/respiratory-vaccines-cannot-work
[33] M Belderbos, M Houben, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Jun 1 2011. 127 (6). https://publications.aap.org/pediatrics/article-abstract/127/6/e1513/30023/Cord-Blood-Vitamin-D-Deficiency-Is-Associated-With
[34] B Graham, L Anderson. Challenges and opportunities for respiratory syncytial virus vaccines. May 24 2013.
[35] C Huber. Ivermectin is safe and effective: The evidence. Sep 9 2021. The Defeat of COVID. https://colleenhuber.substack.com/publish/posts/detail/41112662
[36] COVID-19 Ivermectin Studies. Ivermectin for COVID-19: 105 studies with > 200,000 patients. Jun 2025. https://c19ivm.org/
[37] K Yesilbag, E Toker, et al. Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro. May 2021. Virus Res. 297. 198384. https://www.sciencedirect.com/science/article/abs/pii/S0168170221000915











It’s doubtful ACIP will reverse its decision though. And Malone voted yes… so shameful, but so expected from the likes of him. Never trusted him, from day one. He was squirrelly from the jump.
Thank u for the update . Sadly , they will have blood on their hands . I wish every new parents could read this .